The Interop Council's roadmap forms the roadmap for the next two years and thus the basis of the Interop Council's work. It identifies and prioritises suitable measures to achieve the Council's long-term objectives: Shaping better medicine through greater interoperability.
Roadmap
2023-2024
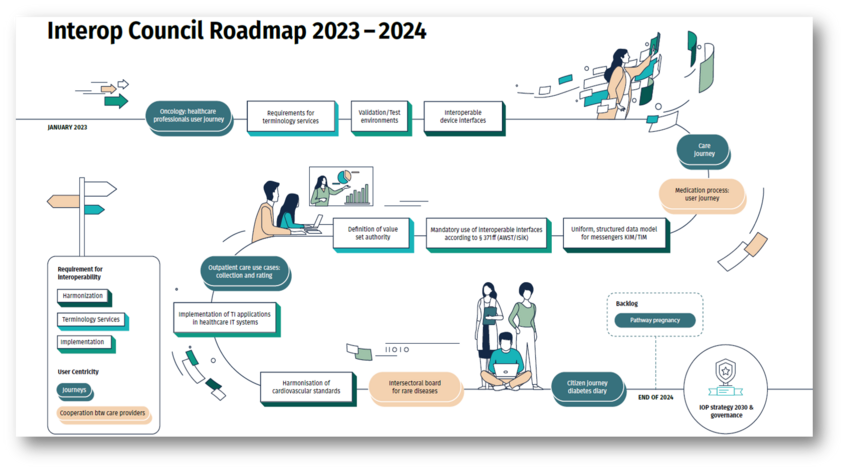
Roadmap of the Interop Council 2023-2024
Dynamic roadmap
The roadmap is a document that is subject to continuous development and annual revision. The roadmap is not a national strategy, but a work programme that focuses on pragmatic feasibility. Nevertheless, a national interoperability strategy and governance for interoperability in Germany should be developed at the end of the first two years of the roadmap.
The topics in detail
The 15 topics on the roadmap have been clustered into six different categories. Enclosed you will find the topic clusters and the individual profiles of the topics.
The content and chronological order of the profiles is dynamic and will adapt to changing conditions.
- Intersectoral co-operation
- Jorneys & Pathways
- Terminology services
- Implementation
- Hamonisation
- Strategies 2030 & Governance
| Specialist user journey medication process | Description/objective: To visualise the medication process holistically and across sectors, taking into account international standards
Benefit/added value: Increase in drug therapy safety, cross-sector data exchange
Operationalisation/implementation: Working group, development of recommendations for action for harmonisation or recommendation of certain standards
Required experts / collaboration: HL7, MIO42, ePA, e-prescription, pharmacists (also inpatient) Timeframe: HY2 2023 |
| Intersectoral board for rare diseases | Description/goal: -
Benefit/added value: Cross-practitioner analysis of data to identify suspected cases of rare diseases and support their treatment, similar to tumour boards
Operationalisation/implementation: Working group, development of cross-sector journey
Required experts / collaboration: Outpatient + inpatient doctors + ? Period: HY 2 2024 |
| Oncological specialist user pathway | Description/goal: Specialist user journey for service providers in oncology (indication tbd)
Benefit/added value: Identification of harmonisation potential in oncology from the perspective of service providers
Operationalisation/implementation: Working group, recommendations for action
Required experts / collaboration: Oncology outpatient & inpatient service providers Period: HY 2 2022 |
| Care Journey | Description/goal: Development of a journey for users from the areas of home care, social care centres and discharge management
Benefit/added value: Identification of interoperability gaps and necessary standardisation tasks.
Operationalisation/implementation: Recommendations for standardisation activities in the care sector
Required experts / collaboration: Nursing staff / nursing experts, nursing IT manufacturers Timeframe: 4 months from March 2023 |
| Collection + evaluation of outpatient use cases | Description/objective: Identification and evaluation of use cases in outpatient care that would gain significant added value through interoperability
Benefit/added value: Based on this, strategies can be developed on how interoperability can be implemented more strongly in outpatient care.
Operationalisation/implementation: Working group, recommendations for action
Required experts / collaboration: outpatient service providers, Mio42/ KBV, gematik Period: HY1 2024 |
| Citizen pathway diabetes diary | Description/objective: Development of a citizen pathway using the example of a diabetes diary
Benefit/added value: Identification of the added value of interoperability for citizens
Operationalisation/implementation: Working group, recommendations for action
Required experts / collaboration: gematik, Mio42, DDG/ diabetes experts Timeframe: HY 2 2024 |
| Terminology server/services | Description/goal: Development of criteria for infrastructure, access, maintenance, services for central terminology servers together with BfArM and gematik
Benefit/added value: Ease of use, better dissemination of international terminologies in DE
Operationalisation/implementation: Recommendations for action for BfArM and gematik
Required experts / collaboration: BfArM, gematik, terminology experts, users and developers
Timeframe: November 2022 - February 2023 |
| Define value set authority | Description/goal: Development of governance for value set providers under the aegis of a value set authority
Benefit/added value: Clear rules for setting up and maintaining coding systems and value lists
Operationalisation/implementation: working group, recommendations for action
Required experts / collaboration: value set provider, BfArM, BMG
Timeframe: HY2 2023 |
| Validation/ test environments | Referenzvalidator | Description/objective: Based on the reference validator provided by gematik, the aim is to work out how and for what purposes future test modules will be made available on a binding basis
Benefit/added value: Test modules can be used to evaluate the correct implementation of applications.
Operationalisation/implementation: Working group, recommendations for action
Required experts/collaboration: Interface development, standardisation, FHIR, primary system development
Timeframe: November 2022 - February 2023 |
| Test environments | Description/Objective: Development of criteria for the design of test environments for testing new (TI) applicationsBenefit/added value: There is currently no possibility to test new applications in various primary systems in advance.
Benefit/added value: There is currently no possibility to test new applications in various primary systems in advance.
Operationalisation/implementation: Working group, recommendations for action
equired experts/cooperation: Primary system manufacturers, specifiers
Timeframe: HY1 2023 | |
| Implementation of TI applications in primary systems | Description/objective: The realisation of TI applications in primary systems is stalling. Various possible solutions (confirmation procedures, control options in productive operation) are to be discussed.
Benefit/added value: Identify acceleration potential for the implementation of TI applications.
Operationalisation/implementation: Working group, recommendations for action
Collaboration/experts required: Gematik, primary system manufacturers, providers of TI applications
Timeframe: HY2 202 | |
| Binding nature of the interoperable interfaces in accordance with §371 and §373 | Liability ISiK | Description/objective: Investigation of the current status of the binding nature of ISiK. Identification of acceleration potential
Benefit/added value: Accelerate and strengthen the use of standards in the stationary sector..
Operationalisation/ implementation: working group, recommendations for action
Required experts/cooperation: primary system manufacturers, specialised users, specifiers
Period: HY2 2023 |
| Liability AWST | Description/objective: To analyse the current status of the liability of AWST.
Benefit/added value: Accelerate and strengthen the use of standards in the outpatient sector.
Operationalisation/implementation: working group, recommendations for action
Required experts/cooperation: KBV, primary system manufacturers, specifiers
Period: HY1 2023 | |
| Interoperable device interfaces | Description/goal: Development of a FHIR-based device interface
Benefit/added value: Replacement of proprietary solutions from the outpatient sector, internationally reusable interface for industry
Operationalisation/implementation: Development of criteria for FHIR interface, if possible commissioning of service provider for development
Required experts/cooperation: device manufacturer, PVS manufacturer
Timeframe: 3 months from February 2023 |
| KIM/ TIM: standardised (structured) data model | Description/objective: Development of criteria and requirements for a standardised data model as a construction kit for data objects in KIM and TIM
Benefit/added value: Dispatch of data in KIM/ TIM (incl. standardisation and structuring).
Operationalisation/implementation: Working group, recommendations for action
Required experts/cooperation: KBV, gematik, manufacturers of TI applications and primary systems, specialist users
Timeframe: HY2 2023 |
| Harmonisation of cardiological standard | Description/objective: Implementation of the working group's recommendations for action (analysis of the status quo of the "heart failure patient journey".
Benefit/added value: still to be defined
Operationalisation/implementation: still to be defined
Required experts/cooperation: still to be defined
Timeframe: HY2 2024 |
| Strategy 2030 & Governance | Description/goal: Continuous development of an IOP strategy and IOP governance for Germany
Benefit/added value: Establishment of clear objectives and rules for cooperation between IOP stakeholders in the German healthcare system.
Operationalisation/implementation: still to be defined
Required experts/cooperation: still to be defined
Timeframe: continuous 2023 - 2024 |
